Abstract
Background: Phospholipase C beta 2 (PLCB2) encodes a phosphodiesterase that catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to the second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). These second messengers modulate the activity of downstream proteins important for cellular signaling. IP3 is soluble and diffuses through the cytoplasm and interacts with IP3 receptors in the endoplasmic reticulum, releasing calcium and raising the level of intracellular calcium. In addition, nuclear factor kappa B can regulate the transcription of this PLCB2, whose protein product is also an important regulator of platelet responses. Until now, the up-regulation of PLCB2 expression has been reported to be associated with the granulocytic differentiation of normal and malignant progenitors and predictor of in vivo responsiveness to all trans-retinoic acid (ATRA) of acute promyelocytic leukemia (APL) patients. In this study, we evaluated the clinical implication of PLCB2 expression in normal karyotype acute myeloid leukemia (NK-AML).
Methods: Using immunohistochemical staining (IHCS) on formalin-fixed, paraffin-embedded (FFPE) bone marrow sections, the expressions of PLCB2 protein were assessed in 101 patients who newly diagnosed as NK-AML. FFPE BM sections measuring 3 μm were deparaffinized in xylene, rehydrated with ethanol, and rinsed in PBS. After deparaffinization at 65 °C for 10 min, the slides were inserted to a Benchmark GX automatic stainer (Ventana Medical Systems, USA). Rabbit polyclonal anti-human PLCB2 antibody (ab176012, Abcam Inc, USA) was applied at 1:50 dilution. Monoclonal rabbit antihuman IgG1-4 antibody (EPR4421, Abcam Inc, USA) at a dilution of 1:500 was used as a negative isotype control. PLCB2 immunostained slides were scored as follows; a brown granular cytoplasmic staining was considered to be positive. The positivity was scored on a scale of 0-8, the sum of a proportion score (0-5) and an intensity score (0-3). The final positivity score was determined as a median of scores by three independent experts. Overall survival (OS) and event free survival (EFS) were analyzed with each cutoff as positivity score. After determining the optimal cutoff value in survival analysis, we compared the results with those of previously well-known prognostic markers: NPM1 mutation and FLT3-ITD, BAALC and WT1 expressions.
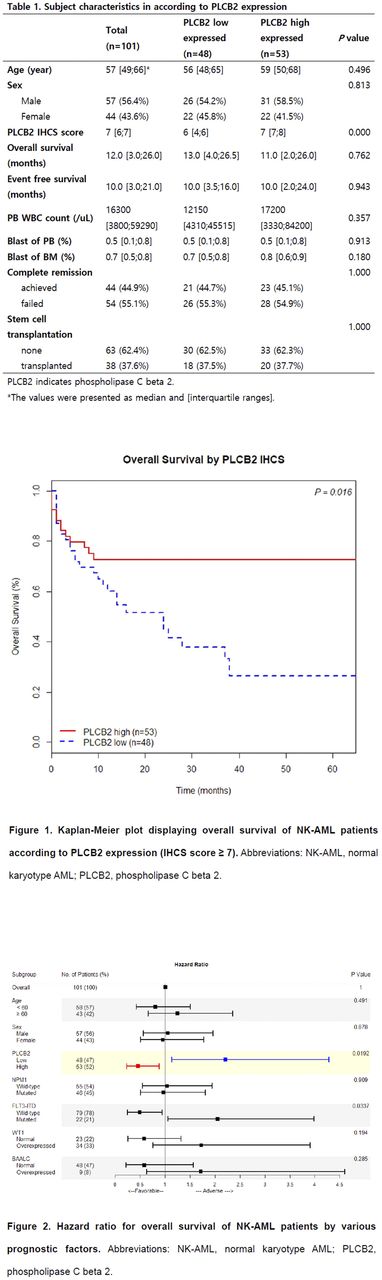
Results: The median age of enrolled patients was 57 (23-83) at the diagnosis. The ratio of male:female was 1:0.77 (57:44). Using the cutoff as positivity score 7.0, PLCB2 high-expressed group showed superior OS (72.6% vs 26.5%; P= 0.016) and low hazard ratio (0.453; P= 0.019) compared to PLCB2 low-expressed group (Figure 1). In the event free survival, PLCB2 high-expressed group showed no significant survival gain (50.6% vs 43.0%, P= 0.465) and hazard ratio (0.7357; P= 0.464). High and low expressed groups of PLCB2 IHCS at diagnosis, were not significantly different in age, sex, WBC count in peripheral blood, blast percentage of peripheral blood and bone marrow, the achievement rate of complete remission, and the enforcement of stem cell transplant (Table 1). Though, among the previously well-known prognostic markers, only positive FLT3 -ITD showed significantly low overall survival rate (29.1% vs negative group 52.7%; P= 0.032) and high hazard ratio (2.052; P= 0.034) (Figure 2).
Conclusions: Overexpression of PLCB2 was associated with favorable OS in NK-AML patients. This study suggests that expression of PLCB2 protein assessed using IHCS is a feasible factor to predict the disease activity and prognosis in NK-AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal